


This page provides supplementary data about the noble gases, which were excluded from the main article to conserve space and preserve focus. Oganesson mostly not included due to the amount of research known about it.
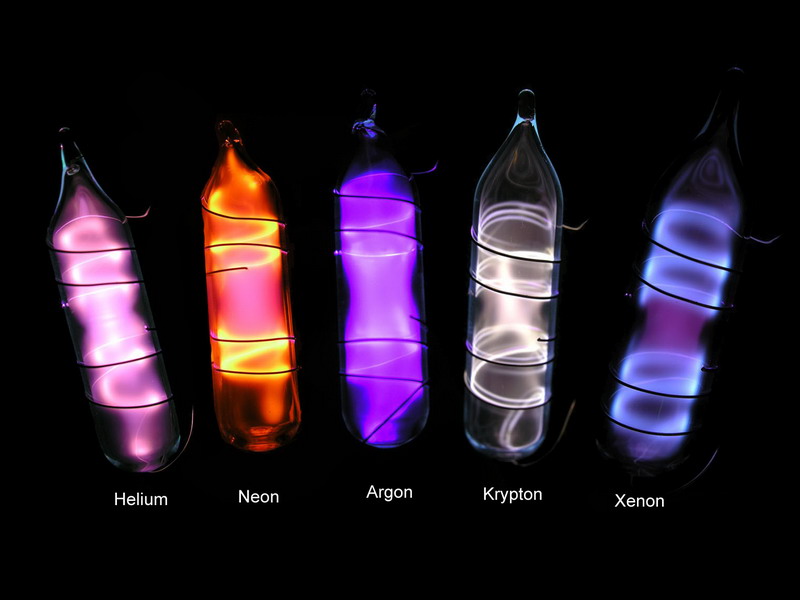
Helium is a non-toxic and non-combustible gas that is not harmful to humans or the environment. The safety of its employees and the communities in which it operates is always Freeport LNG’s highest priority. Freeport LNG is committed to the safe construction and operation of all its facilities. Helium is a noble gas because it is extremely difficult to entice it into compounding with anything. Once it gets its two customary electrons it is chemically satisfied. It’s a gas because its triple point is very low - so low that it doesn’t form a solid even under pressure. Noble gas, any of the seven chemical elements that make up Group 18 (VIIIa) of the periodic table. The elements are helium (He), neon (Ne), argon (Ar), krypton (Kr), xenon (Xe), radon (Rn), and oganesson (Og). Are noble gases flammable? Helium is one of the most common elements in the universe. It is called a noble gas because it doesn’t chemically interact with elements. Its atomic number is 2 and the weight is 4.002. In its natural state, it doesn’t have any smell, taste or color. Common Uses of Helium Evidence shows that the human voice can be changed with a bit of helium. The gas is also used as light weight aircraft.

Physical properties[edit]
Solid[edit]
| Physical property | Helium | Neon | Argon | Krypton | Xenon | Radon |
|---|
| Density, solid at tp (g/dm³)[1] | – | 1444 | 1623 | 2826 | 3540 | – |
| Crystal structure[2] | hcp | fcc | fcc | fcc | fcc | fcc |
Liquid[edit]
| Physical property | Helium | Neon | Argon | Krypton | Xenon | Radon |
|---|
| Density, liquid at bp and 1 atm (g/dm³)[1] | 125.0 | 1207 | 1393.9 | 2415 | 3057 | 4400 |
| Density, liquid at tp (g/dm³)[1] | – | 1247 | 1415 | 2451 | 3084 | – |
| Thermal conductivity, liquid at bp (mW m−1 K−1)[1] | 31.4 | 129.7 | 121.3 | 88.3 | 73.2 | – |
| Dielectric constant (liquid)[3][4] | 1.057[5][6] | 1.191[7][8] | 1.325 [9] | 1.664[10] | 1.880[11] | — |
Gas[edit]
| Physical property | Helium | Neon | Argon | Krypton | Xenon | Radon |
|---|
| Density, gas at 0 °C and 1 atm (g/dm³)[2] | 0.1786 | 0.9002 | 1.7818 | 3.708 | 5.851 | 9.97 |
| Thermal conductivity at 0 °C (J s−1 m−1 K−1)[12] | 0.1418 | 0.0461 | 0.0169 | 0.00874 | 0.00506 | 0.0036[13] |
| Mean Free Path at STP (nm)[2] | 192.66 | 135.36 | 68.33 | 52.34 | 37.88 | – |
| Solubility in water at 20 °C (cm3/kg) [12] | 8.61 | 10.5 | 33.6 | 59.4 | 108.1 | 230 |
| Magnetic susceptibility (cgs units per mole)[2] | −0.0000019 | −0.0000072 | −0.0000194 | −0.000028 | −0.000043 | – |
| Heat capacity, Cp, gas at 1 atm (J mol−1 K−1)[1] | 20.78 | 20.79 | 20.85 | 20.95 | 21.01 | 21 |
| Sonic velocity at 0 °C and 1 atm (m/s)[1] | 973 | 433 | 307.8 | 213 | 168 | – |
| Thermal conductivity, gas at 0 °C and 1 atm (mW m−1 K−1)[1] | 141.84 | 46.07 | 16.94 | 8.74 | 5.06 | 3.6[13] |
| Molar refraction (D line, cm3)[14] | 0.521 | 1.004 | 4.203 | 6.397 | 10.435 | – |
| Dielectric constant (gas)[15] | 1.0000684[16] | 1.00013[17] | 1.000516[18] | – | – | – |
| van der Waals constanta (L2bar/mol2)[15] | 0.03412 | 0.2107 | 1.345 | 2.318 | 4.194 | – |
| van der Waals constantb (L/mol)[15] | 0.02370 | 0.01709 | 0.03219 | 0.03978 | 0.05105 | – |
Phase changes and critical properties[edit]
| Physical property | Helium | Neon | Argon | Krypton | Xenon | Radon |
|---|
| Boiling point (°C)[2] | −268.8 | −245.9 | −185.8 | −151.7 | −106.6 | −61.7 |
| Boiling point (K) | 4.15 | 27.15 | 87.15 | 121.2 | 165.2 | 211.3 |
| Melting point (°C)[2] | −272 | −248.5 | −189.6 | −157.4 | −111.5 | −71.0 |
| Critical temperature (K)[2] | 5.25 | 44.5 | 150.85 | 209.35 | 289.74 | 378.15 |
| Critical pressure (atm)[2] | 2.26 | 26.9 | 48.3 | 54.3 | 57.64 | 62 |
| Critical density (g/mL)[2] | 0.0693 | 0.484 | 0.536 | 0.908 | 1.100 | – |
| Triple point temperature (K)[1] | 2.19[19] | 24.562 | 83.80 | 115.76 | 161.37 | 202 |
| Triple point pressure (kPa)[1] | 5.1[19] | 43.37 | 68.90 | 73.15 | 81.66 | 70 |
Atomic properties[edit]
| Atomic property | Helium | Neon | Argon | Krypton | Xenon | Radon | Oganesson |
|---|
| Atomic number[12] | 2 | 10 | 18 | 36 | 54 | 86 | 118 |
| Standard atomic weight[12] | 4.002602(2) | 20.1797(6) | 39.948(1) | 83.80(1) | 131.29(2) | (222) | (294) |
| Number of natural isotopes[12] | 2 | 3 | 3 | 6 | 9 | 4 | 0 |
| Outer shell electron configuration[12] | 1s2 | 2s22p6 | 3s23p6 | 4s24p6 | 5s25p6 | 6s26p6 | 7s27p6 |
| Atomic radius (pm)[2] | 31 | 38 | 71 | 88 | 108 | 120 | 138 |
| Ionization energy (kJ/mol)[12] | 2372 | 2080 | 1520 | 1351 | 1170 | 1037 | 839 |
| Static polarizability[2] (Å3) | 0.204 | 0.392 | 1.63 | 2.465 | 4.01 | – | – |
| Average Valence Electron Energy (AVEE) | 4.16 | 4.79 | 3.24 | 2.97 | 2.58 | 2.60 | – |
Helium Noble Gas Meme
Abundance[edit]
| Abundance | Helium | Neon | Argon | Krypton | Xenon | Radon | Oganesson |
|---|
| Solar System (for each atom of silicon)[20] | 2343 | 2.148 | 0.1025 | 5.515 × 10−5 | 5.391 × 10−6 | – | – |
| Earth's atmosphere (volume fraction in ppm)[21] | 5.20 | 18.20 | 9340.00 | 1.10 | 0.09 | (0.06–18) × 10−19 | – |
| Igneous rock (mass fraction in ppm)[12] | 3 × 10−3 | 7 × 10−5 | 4 × 10−2 | – | – | 1.7 × 10−10 | – |
Economic data[edit]
| Gas | 2004 price (USD/m3)[1] |
|---|
| Helium (industrial grade) | 4.20–4.90 |
| Helium (laboratory grade) | 22.300–44.90 |
| Argon | 2.70–8.50 |
| Neon | 60–120 |
| Krypton | 400–500 |
| Xenon | 4000–5000 |
Radon is available only in very small quantities, and due to its short half-life, is generally produced by a radium-226 source in secular equilibrium.[22] Oganesson is almost impossible to produce and with a very short half life, it is generally not readily available for purchase.
Helium Noble Gas Notation
References and notes[edit]

- ^ abcdefghijShuen-Chen Hwang; Robert D. Lein; Daniel A. Morgan (2005). 'Noble Gases'. Kirk Othmer Encyclopedia of Chemical Technology. Wiley. pp. 343–383. doi:10.1002/0471238961.0701190508230114.a01. ISBN978-0471238966.
- ^ abcdefghijk'Noble Gas'. Encyclopædia Britannica. 2008.
- ^Amey, R. L. (1964). 'Dielectric Constants of Liquefied Noble Gases and Methane'. Journal of Chemical Physics. 40 (1): 146–148. Bibcode:1964JChPh..40..146A. doi:10.1063/1.1724850.
- ^CRC handbook of chemistry and physics : a ready-reference book of chemical and physical data. Haynes, William M., Lide, David R., 1928-, Bruno, Thomas J. (2016-2017, 97th ed.). Boca Raton, Florida. 2016-06-22. ISBN978-1-4987-5429-3. OCLC957751024.CS1 maint: others (link)
- ^at 1.5–2.5 K
- ^Chase, C.E.; Maxwell, E.; Millett, W.E. (December 1961). 'The dielectric constant of liquid helium'. Physica. 27 (12): 1129–1145. Bibcode:1961Phy....27.1129C. doi:10.1016/0031-8914(61)90054-4.
- ^at 26.11 K
- ^'Dielectric Constant | The Elements Handbook at KnowledgeDoor'. KnowledgeDoor. Retrieved 2019-12-17.
- ^at 140 K
- ^at 119.80K
- ^at 161K
- ^ abcdefghGreenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN978-0-08-037941-8.
- ^ abGeneralic, Eni,'Radon,' EniG. Periodic Table of the Elements. 27 May 2013. KTF-Split. (accessed 30 May 2013).
- ^Peter Häussinger; Reinhard Glatthaar; Wilhelm Rhode; Helmut Kick; Christian Benkmann; Josef Weber; Hans-Jörg Wunschel; Viktor Stenke; Edith Leicht; Hermann Stenger (2002). 'Noble gases'. Ullmann's Encyclopedia of Industrial Chemistry. Wiley. doi:10.1002/14356007.a17_485. ISBN978-3527306732.
- ^ abcLide, D. R. (Ed.) (1990). CRC Handbook of Chemistry and Physics (70th Edn.). Boca Raton (FL):CRC Press.CS1 maint: extra text: authors list (link)
- ^<3 × 106 Hz at 140 °C
- ^106 Hz at 0°C
- ^1015 Hz at 20°C
- ^ abLambda point for pure 4He from Yunus A. Cengel, Robert H. Turner. Fundamentals of thermal-fluid sciences. McGraw-Hill, 2004, p. 78. ISBN0-07-297675-6
- ^Lodders, Katharina (July 10, 2003). 'Solar System Abundances and Condensation Temperatures of the Elements'(PDF). The Astrophysical Journal. 591 (2): 1220–1247. Bibcode:2003ApJ...591.1220L. doi:10.1086/375492. Archived from the original(PDF) on November 7, 2015. Retrieved September 2, 2015.
- ^'The Atmosphere'. National Weather Service. Retrieved 2008-06-01.
- ^Collé, R; Kishore, Raj (1997-06-11). 'An update on the NIST radon-in-water standard generator: its performance efficacy and long-term stability'. Nuclear Instruments and Methods in Physics Research Section A: Accelerators, Spectrometers, Detectors and Associated Equipment. 391 (3): 511–528. Bibcode:1997NIMPA.391..511C. doi:10.1016/S0168-9002(97)00572-X.
Helium Exploration Companies
Retrieved from 'https://en.wikipedia.org/w/index.php?title=Noble_gas_(data_page)&oldid=1016802349'






