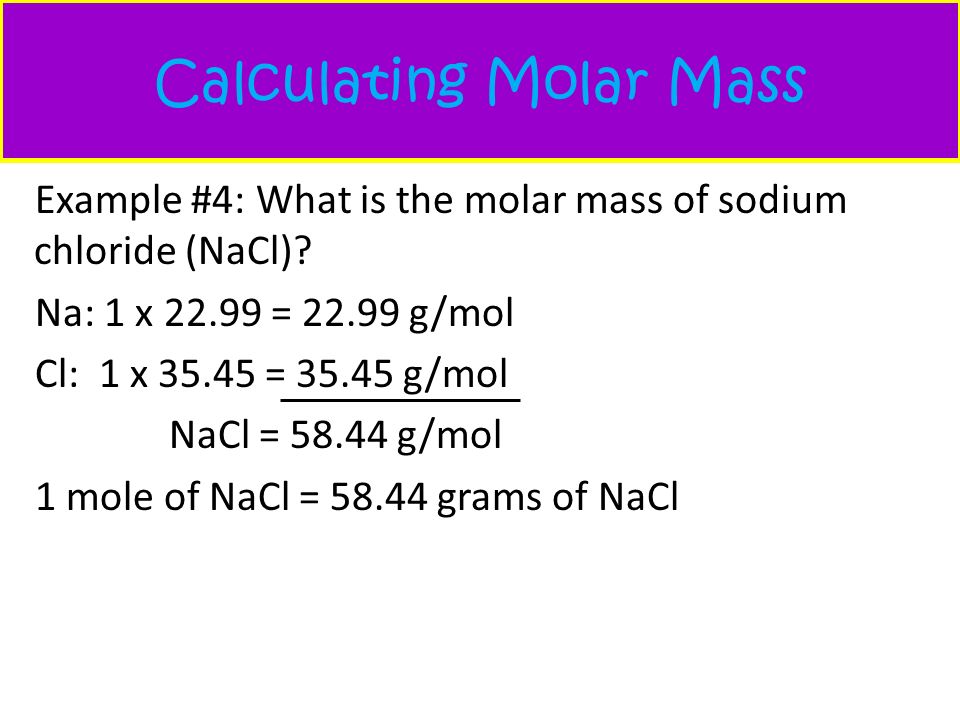
How to find the Molecular Weight for NaCl (sodium chloride): a step-by-step video tutorial. Using the Periodic Table look up the atomic weight of each of th. NaCl is a compound, so it does not have an atomic mass. In order to derive the molar mass, add up the atomic mass of the atoms in the formula: Atomic mass Na = 22.990amu Atomic mass Cl = 35.453 amu. . Molar mass is the amount grams that one mole weighs. You need to find the molar mass of NaCl which is the same as the amu on the periodic table in grams. So it is 22.99(Na) + 35.45(Cl) = 58.44 You also know that for every mole of NaCl you have. ›› NaCl molecular weight. Molar mass of NaCl = 58.44277 g/mol. This compound is also known as Sodium Chloride. Convert grams NaCl to moles or moles NaCl to grams. Molecular weight calculation: 22.98977 + 35.453 ›› Percent composition by element.
Solving for the atomic mass of sodium chloride (NaCl)
Need to know the atomic mass of a sodium chloride molecule? Our molar mass calculator uses the periodic table and the chemical formula to solve for the molar mass of a chemical compound based on the compound's empirical formula. The calculator takes the elemental composition of the compound and weighs the elements to get an empirical formula mass. Note that the calculator assumes a pure substance - if you're aware of dilution or impurities, make appropriate adjustments for the molarity of a given substance.
This project started with as a molar mass calculator for chemical reactions. You can use our calculator to solve for the theoretical yield of an experiment. We also have a percent yield calculator which can help you apply this to actual experiments. Use the mole ratio and empirical formula to understand the limits of the reactants.
Other terms: atomic mass of sodium chloride, molar mass of sodium chloride, molecular mass, Adobe photoshop lightroom classic cc 2018 free download mac.

How Does The Molar Mass Calculator Work?
We take the formula you provide (NaCl - common table salt - in our default example) and unpack it into the component elements. Then we compare each atom against a table of the standard atomic weights for that element. We present the results in a table at the bottom of the molar mass calculator - it will show the count of atoms, the atomic weight of each element, and the molecular weight for the molecule. It solves for total mass of a molecular formula (average molecular weight).
From there we break the formula for sodium chloride into parts - a Sodium atom, a Chlorine atom, etc.
We don't have brackets implemented (yet), so you will need to unpack any bracketed expressions. They don't affect the weight anyhow. Simply take each element and multiple it by the number of times the bracketed structure occurs. For example: (C6H5)3PCCO => C18H15PCCO
Finding Molar Mass for Other Chemical Compounds
Our molar mass calculator has this for a variety of other compounds: sodium chloride, carbon dioxide, sulfuric acid, glucose..
Bookmarking, Save, and Share Results
The tool is designed so you can flip between different parts of a problem set. We recommend you bookmark it so you can refer back to it. You can also share results with a study partner or tutor by hitting calculate and copying the URL for this page. When your study partner opens up the URL, they will see your calculations. It's easy share & save results via email. (Be sure to hit calculate first, however)

Nacl Atomic Mass Chart
You also have the option of saving links to the calculations in your research notes files, so you can quickly re-open or check them later. Again - hit calculate first so the URL is updated with your most recent changes. Then copy and save the url.
FAQ - Molar Mass Calculator
What is Molar Mass in Chemistry?
Molar mass is an important concept in adapting chemical formulas to real world conditions. We may be able to balance a chemical equation and determine that one molecule of hydrogen combines with two of oxygen to make water (or the compound of your choice). But how would you set up the materials in the laboratory? Or if you were, for example, buying oxygen for a process, how would you determine how much to use to make a given quantity of water? Molar mass allows us to convert a chemical reaction into specific amounts of reagents required for the process. By converting the atomic interaction into grams, we can measure and use an appropriate amount of the necessary reagents. Formula mass helps us solve for this.
What Is Relative Atomic Mass / Relative Molecular Mass / Average Molecular Weight?
The relative atomic mass of a compound is the ratio of the average mass of the elements in a chemical compound to the atomic mass constant, which is defined as 1/12 the mass of a carbon 12 atom. For a single sample, the relative atomic mass of the sample is the weighted arithmetic mean of the masses of the individual atoms present in the sample (also known as the average atomic mass). This will vary by isotope of the element (carbon-12 vs. carbon-13, for example, since the two isotopes have a different atomic mass due to additional neutrons). In the real world, this can vary based on where the sample was collected - due to variances in the specific isotopes of the elements present (driven by differences in radioactive decay and how the material was aggregated to begin with).
Nacl Atomic Mass Definition
How to find Molar Mass
Nacl Relative Atomic Mass
Take a standard chemistry formula for a molecule, split it up into the component atoms, and look up the molar weight of each atom. Add the weight of the atoms in the molecule and you have the molar mass for the molecule.
