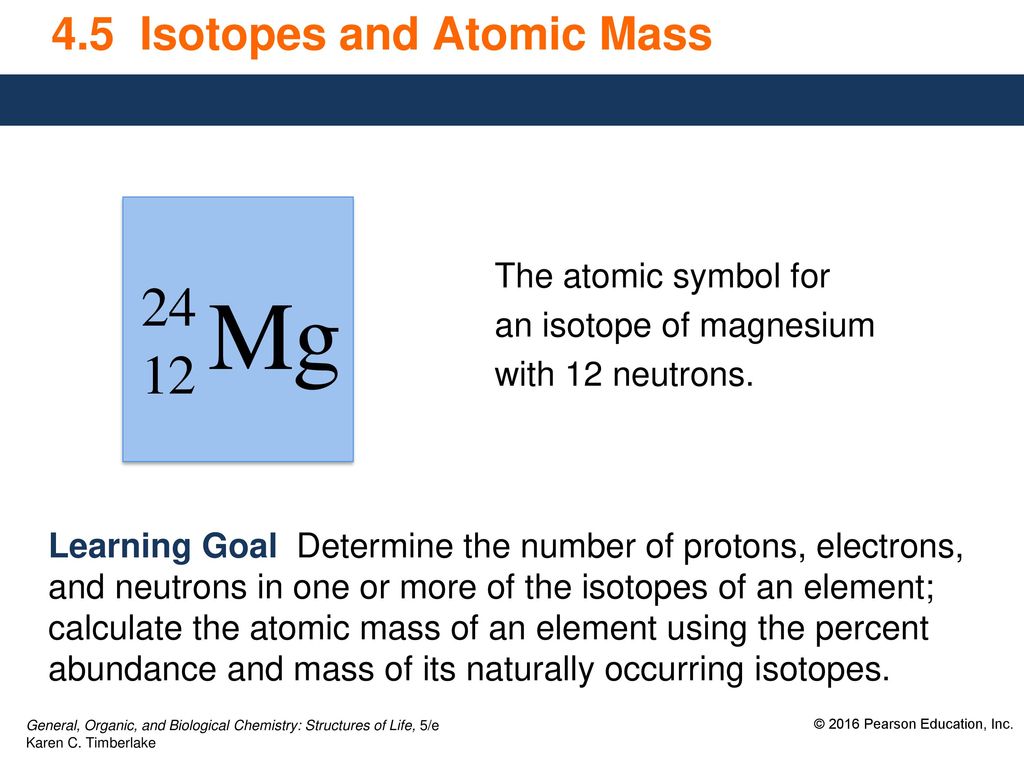
| Isotope | Atomic mass (Da) | Isotopic abundance (amount fraction) |
|---|---|---|
| 24Mg | 23.985 041 70(9) | [0.7888, 0.7905] |
| 25Mg | 24.985 8370(3) | [0.099 88, 0.100 34] |
| 26Mg | 25.982 5930(2) | [0.1096, 0.1109] |
In 2011, the Commission has changed the standard atomic weight of magnesium to Ar(Mg) = [24.304, 24.307] based on an evaluation of the effect of variation in isotopic abundances in normal materials upon the atomic weight of magnesium. This change is intended to emphasize the fact that the atomic weight of magnesium is not a constant of nature, but depends upon the source of the material. The standard atomic weight was determined by combining (1) the best calibrated isotope-ratio measurement of magnesium in DSM3 isotopic reference material (a mono-elemental nitric solution of magnesium), and (2) the relative isotope-ratio differences between other magnesium-bearing materials and DSM3. Many of the δ26/24Mg measurements reported herein were made using DSM3 as the standard. However, the Commission does not recommend DSM3 as an international measurement standard for δ26/24Mg measurements because it is not readily available to laboratories worldwide.
Atomic weights of the elements 2011 by M.E. Wieser et al. Pure Appl. Chem. 2013 (85) 1047-1078
CIAAW
Magnesium
Ar(Mg) = [24.304, 24.307] since 2011
The name derives from Magnesia, a district in the north-eastern region of Greece called Thessalia. TheScottish chemist Joseph Black recognized it as a separate element in 1755. In 1808, the English chemistHumphry Davy obtained the impure metal, and in 1831 the French pharmacist and chemist Antoine-Alexandre Brutus Bussy isolated the metal in the pure state.
Magnesium is a Group 2 chemical element with symbol Mg & atomic number 12. Know the atomic mass of magnesium, magnesium atomic number and properties of magnesium. Milligram to atomic mass unit (mg—u) measurement units conversion. The SI unit of mass is a kilogram, which is defined by taking the fixed numerical value of the Planck constant h to be 6.626 070 15 × 10⁻³⁴ when expressed in the unit J s, which is equal to kg m² s⁻¹, where the meter and the second are defined in terms of c and Δν Cs. Magnesium is a chemical element with the symbol Mg and atomic number 12. It is a shiny gray solid which bears a close physical resemblance to the other five elements in the second column (group 2, or alkaline earth metals) of the periodic table: all group 2 elements have the same electron configuration in the outer electron shell and a similar crystal structure.
Mg-24 Atomic Mass



Mg-26 Atomic Mass
Natural variations of magnesium isotopic composition
Mg Atomic Weight
Isotopic reference materials of magnesium.
