Introduction
Chiral Carbon Example
The opposite of chiral is achiral. Achiral objects are superimposable with their mirror images. If the molecules are superimposable, they are identical to each other. For example, two pieces of paper are achiral. In contrast, chiral objects, like our hands, are non-superimposable mirror images of each other. Try to line up your left hand perfectly with your right hand, so that the palms are both facing in the same directions. Spend about a minute doing this. Do you see that they cannot line up exactly?

The same thing applies to some molecules. A chiral molecule has a mirror image that cannot line up with it perfectly - the mirror images are non-superimposable. This pair of non-superimposable mirror image molecules are called enantiomers. But why are chiral molecules so interesting? Just like your left hand will not fit properly in your right glove, one of the enantiomers of a molecule may not work the same way in your body, as the other. It turns out that many of the biological molecules such as our DNA, amino acids and sugars, are chiral molecules.
This must mean that enantiomers have properties that make them different from their mirror image molecule. One of these properties is that chiral molecules do not have a plane ofsymmetry or an internal mirror plane. So, a chiral molecule cannot be divided into two identical halves. Another property of chiral molecules is optical activity.
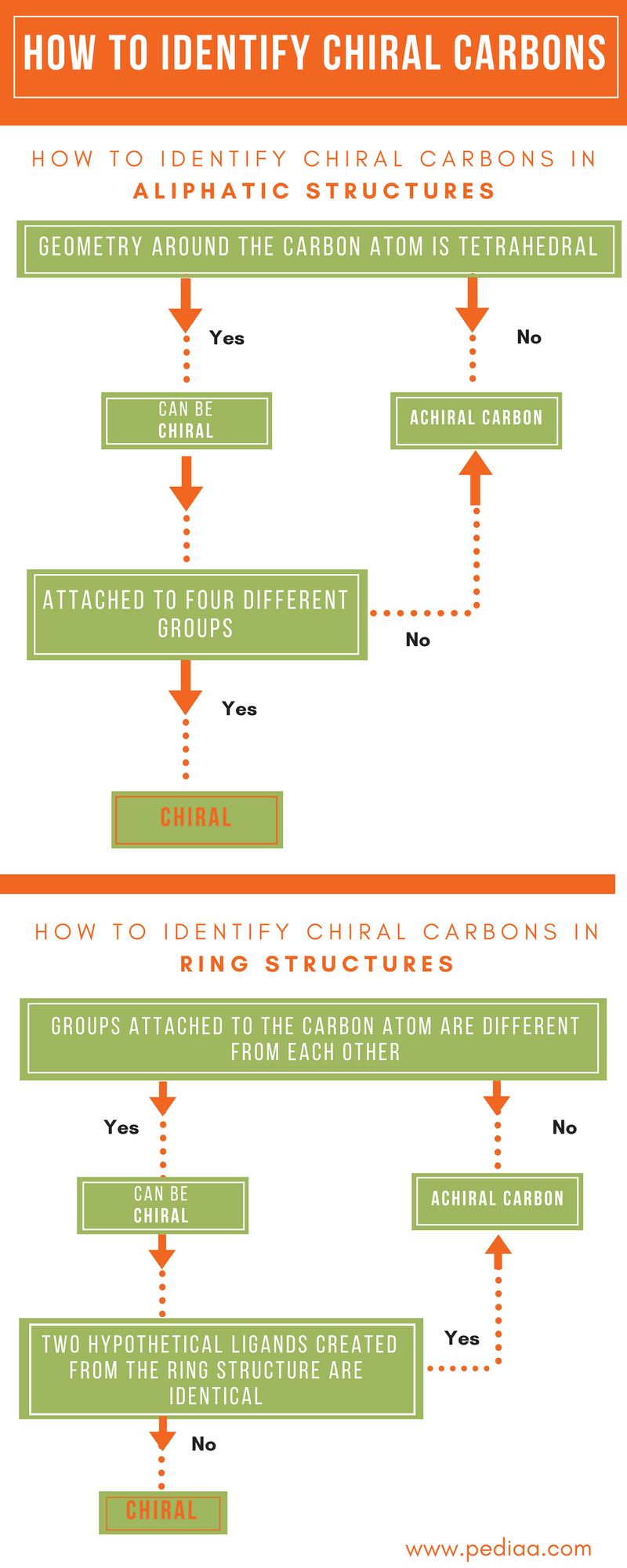
- Chiral centers are tetrahedral atoms (usually carbons) that have four different substituents. Each chiral center in a molecule will be either R or S. As noted above, molecules with a single chiral center are chiral. Molecules with more than one chiral center are usually chiral.
- Chiral vector C can be written as C = n a 1 + m a 2 where a 1 and a 2 are basis vectors of the graphene lattice. The pair of integers (n,m) is called the chiral index or just chirality. This implies that the structure of a single-walled carbon nanotube is completely determined by chirality.
Organic compounds, molecules created around a chain of carbon atoms (more commonly known as the the carbon backbone), play an essential role in the chemistry of life. Free beat making software for mac. These molecules derive their importance from the energy they carry, mainly in a form of potential energy. Since potential energy can be widely affected due to changes in atomic placement, it is important to understand the concept of an isomer, a molecule sharing the same atomic connectivity as another but differing in structural arrangements. This section is devoted to a specific type of isomer called stereoisomers and their property of chirality (Figure 5.1.1).
Archicad 22 mac download. Figure 5.1.1. Two enantiomers of a tetrahedral complex. from Wikipedia
Chiral carbon is the carbon atom about which all the 4 groups are different. Any carbon atom which has 2 groups different can also be called asymmetric but for optical activity to show, all the 4 carbons should be different.
The concepts of steroisomerism and chirality command a great deal of importance in modern organic chemistry, as these ideas help to explain the physical and theoretical reasons behind the formation and structures of numerous organic molecules, the main reason behind the energy embedded in these essential chemicals. In contrast to more well-known constitutional isomerism, which develops isotopic compounds simply by different atomic connectivity, stereoisomerism generally maintains equal atomic connections and orders of building blocks as well as having same numbers of atoms and types of elements.
Chiral Carbon Center
What, then, makes stereoisomers so unique? To answer this question, the learner must be able to think and imagine in not just two-dimensional images, but also three-dimensional space. This is due to the fact that stereoisomers are isomers because their atoms are different from others in terms of spatial arrangement.
